Structures Of Metals And Ceramics
Metals many ceramics some polymers atoms have no periodic packing occurs for.
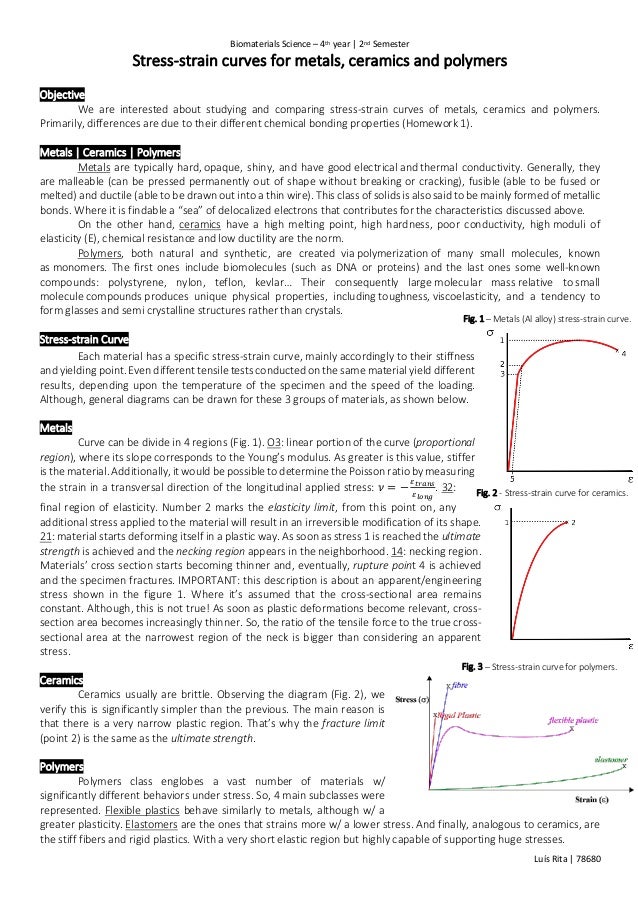
Structures of metals and ceramics. Metal and ceramic crystal structures instructor. The two most common chemical bonds for ceramic materials are covalent and ionic. We ll discuss properties of metals al. Amorphous structure means that atoms are not organized according to a well ordered repeating arrangement as in crystals.
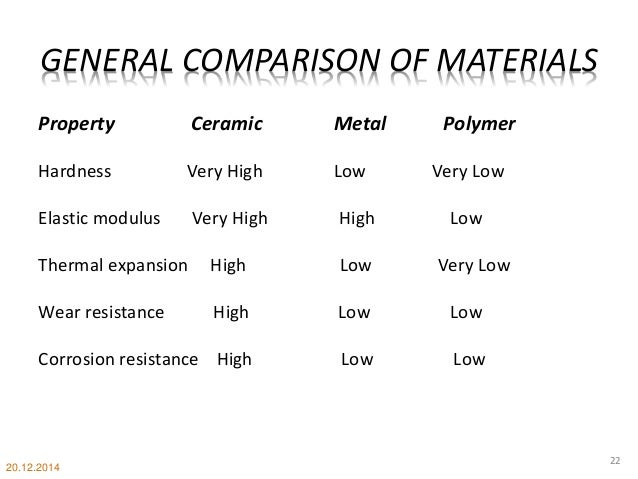
Usually they are metal oxides that is compounds of metallic elements and oxygen but many ceramics. Glass ceramics are made of small grains surrounded by a glassy phase and have properties in between those of glass and ceramics. The table below provides a summary of the main properties of ceramics and glass. The atoms in ceramic materials are held together by a chemical bond.
Noncrystalline materials complex structures rapid cooling si oxygen crystalline sio2 amorphous noncrystalline noncrystalline sio2 adapted from fig. Ceramic composition and properties atomic and molecular nature of ceramic materials and their resulting characteristics and performance in industrial applications. Start studying ch 3. Industrial ceramics are commonly understood to be all industrially used materials that are inorganic nonmetallic solids.
Recall that when metal in the liquid state is cooled a crystalline solid precipitates when the melting freezing point is reached. For metals the chemical bond is called the metallic bond. Materials science engineering ceramic crystal structure 3 3 timeline ce. Today we ll explore more about two of the three main types of materials that we use as engineers.
Structures of metals and ceramics. The bonding of atoms together is much stronger in covalent and ionic bonding than in metallic. Learn vocabulary terms and more with flashcards games and other study tools.